|
 Aki
Kobayashi Aki
KobayashiForensic- Medicine ISSN 1930-6741 |
|
|
| Volume 1 Issue 1 Article # 701 |
 Paper of the Month Introduction
This paper is an excellent basic research in human micro anatomy and
immune staining explaining the type of neurons with the transmitters
which elaborate the areas of our brain function.
This paper is a true research advancement in the understanding of
brain function.
I compliment the authors and our esteemed Editor for publishing
this paper.
Ramesh Kaul, MD, FCCP, M. Surgery.
From Anil Aggrawal's Internet Journal of Forensic Medicine & Toxicology Expression of prostaglandin D2 synthase in human cerebellum and medulla oblongata–An immunohistochemical examination–
The authors are
Aki Kobayashi1, Aiko Takamure1, Izumi
Takase1, Chikako Uemura1, Tokiko Nakagawa1,
Yoshio Yamamoto1, Shigeru Yamasaki1, Akira
Morimoto1, Keiko Ikemoto1, Steven Rand2,
Hedwege Spalthoff3, Beate Annuss3, Iwao
Ohkubo4, and Katsuji Nishi1
1Department of Legal Medicine and 4Department of Medical Biochemistry, Shiga University of Medical Science, Ohtsu 520-2192, Shiga, Japan
2Forensisch DNA-Laboratorium, Universitair Ziekenhuis
Antwerpen, Wilrijkstraat 10, 2650 Edegem, Belgium
Expression of prostaglandin D2 synthase in human
cerebellum and medulla oblongata–
An immunohistochemical examination– Abstract The distribution of prostaglandin D2 synthase (PGDS) in human cerebellum and medulla oblongata was examined using anti PGDS antibody raised and purified in our laboratory. This antibody is specific to PGDS and does not recognize the carbohydrate antigens, Lewis x or sialyl-Lewis x antigens on the PGDS molecule. This antibody was able to stain a large number of the Purkinje cells, neurons in the dentate nucleus in the cerebellum and neurons in the nuclei within the medulla oblongata including nucleus olive, nucleus nervi hypoglossi, nucleus gracilis, nucleus cuneatus and nucleus ambiguous. Since the cerebellum and medulla oblongata, in addition to their functions in equilibrium, motor tone and movement coordination, play critical roles in controlling blood pressure, respiratory patterning and sleeping, the PGDS, which is a major protein in the cerebro-spinal fluid and produces prostaglandin D2 (PGD2), might play an important and fundamental role in the brain functions. Therefore anti-PGDS antibody might become a useful tool for detecting the viability of the Purkinje cells and determination for the assessment the brain damage due to ischemia, obstructive sleep apnea and/or heat stroke in cadavers. Introduction The prostaglandin D2 (PGD2) has long been associated with inflammatory and atopic conditions reacting as an inflammatory mediator and PGD2 also mediates a number of other effects such as inhibition of platelet aggregation, smooth muscle relaxation and contraction, vasodilatation, vasoconstriction and mucus secretion (1). The PGD2 was found to be a major prostaglandin (PG) in the brain of various mammals (2) and to exhibit a lot of neural activities, such as sleep induction (3), body temperature regulation(4) and neuroendocrine functions (5). The binding protein specific to PGD2 localizes in the hypothalamus, amygdala, hippocampus, cerebellar nuclei, thalamus, nucleus accumbens and cerebral cortex in human brain (6). Hoffmann et al (7) purified beta-trace protein which is a major glycoprotein found in human cerebro-spinal fluid and reported that beta-trace protein was identified as the lipocalin-type prostaglandin D2 synthase (L-PGDS). By immunoperoxidase staining with specific polyclonal or monoclonal antibodies and by in situ hybridization with the anti-sense RNA, lipocalin-type prostaglandin D2 synthase which is one of the PGDS in rat (8) and human brain(9) was shown to be mainly produced in the leptomeninges and choroids plexus, rather than in the oligodendrocytes of the parenchyma. The PGDS possesses two N-glycosylation sites of its polyglycopeptide (7). Grabenhorse et al (10) showed that human alpha 1,3/4-fucosyltransferase III-VII were able to synthesize Lewis x and sialyl Lewis x motifs on diantennary N-glycan of beta-trace protein secreted from BHK-21 cells . Lewis x is one of the blood group ABO related antigens and is also called CD 15 or SSEA-I antigen (10). In our previous study Lewis x antigen was intensively expressed in human brain, although sialyl Lewis x antigen was not recognized in the brain except in the anterior lobe of the hypophysis (11). We also purified beta-trace protein from the pooled human cerebrospinal fluid and produced polyclonal antibody against the beta-trace protein (12). Although distribution of PGDS in rat brain was investigated (13) and it was shown that PGD2 plays an important role on sleep regulation in rat (14), the distribution of PGDS and significance of PGD in human brain are not clear yet to date. In the present paper we examined the distribution of PGDS via immunohistochemistry in the cerebellum and medulla oblongata of human brain, since the brainstem may be related with sleeping moderation (15). We also examined the specificity of antibody produced in our laboratory, whether the antibody possess the cross-reactivity with Lewis x.
Materials and Methods Human brain tissues were obtained from 5 male individuals at autopsies carried out at Department of Legal Medicine, Shiga University of Medical Science. Tissue samples were fixed in 10% formalin and embedded in paraffin, and 3-micrometer sections were prepared for immunostaining and immunofluorescent labeling using antibodies to PGDS and Lewis x antigen. Rabbit polyclonal anti PGDS was produced in our department (12), and mouse monoclonal anti Lewis x antibody, Alexa Flour 488 (red) goat anti-mouse IgG and Alexa Flour 568 (green fluorescent) goat anti-rabbit IgG were purchased from DakoCytomation and Invitrogen, respectively. After treating with 0.3% H2O2 in methanol, the sections were incubated for overnight at 4 °C with anti-PGDS (diluted 1:1000) or anti Lewis antibodies (diluted 1: 100), for 2 hours at room temperature with biotinylated anti rabbit IgG and anti mouse IgG, respectively. All the immunochemicals used were diluted in phosphate buffered saline. The color was developed by immersing the section for 10 minutes with a mixture containing 0.02% diaminobenzidine (DAB), 0.0045% H2O2 in Tris-HCl buffer. The stained sections were mounted on glass slide and cover-slipped with Enteran. For the double immunofluorescent labeling, the sections were incubated with phosphate buffer containing anti Lewis x mouse monoclonal antibody and anti-PGDS rabbit polyclonal antibody for overnight at 4 °C. After washing off the primary antibodies, they were incubated with fluorescence labeled anti-mouse IgG and anti rabbit IgG for 2 hours at room temperature. Fluorescence was observed under confocal laser-scanning microscope (LSM510 Carl Zeiss).
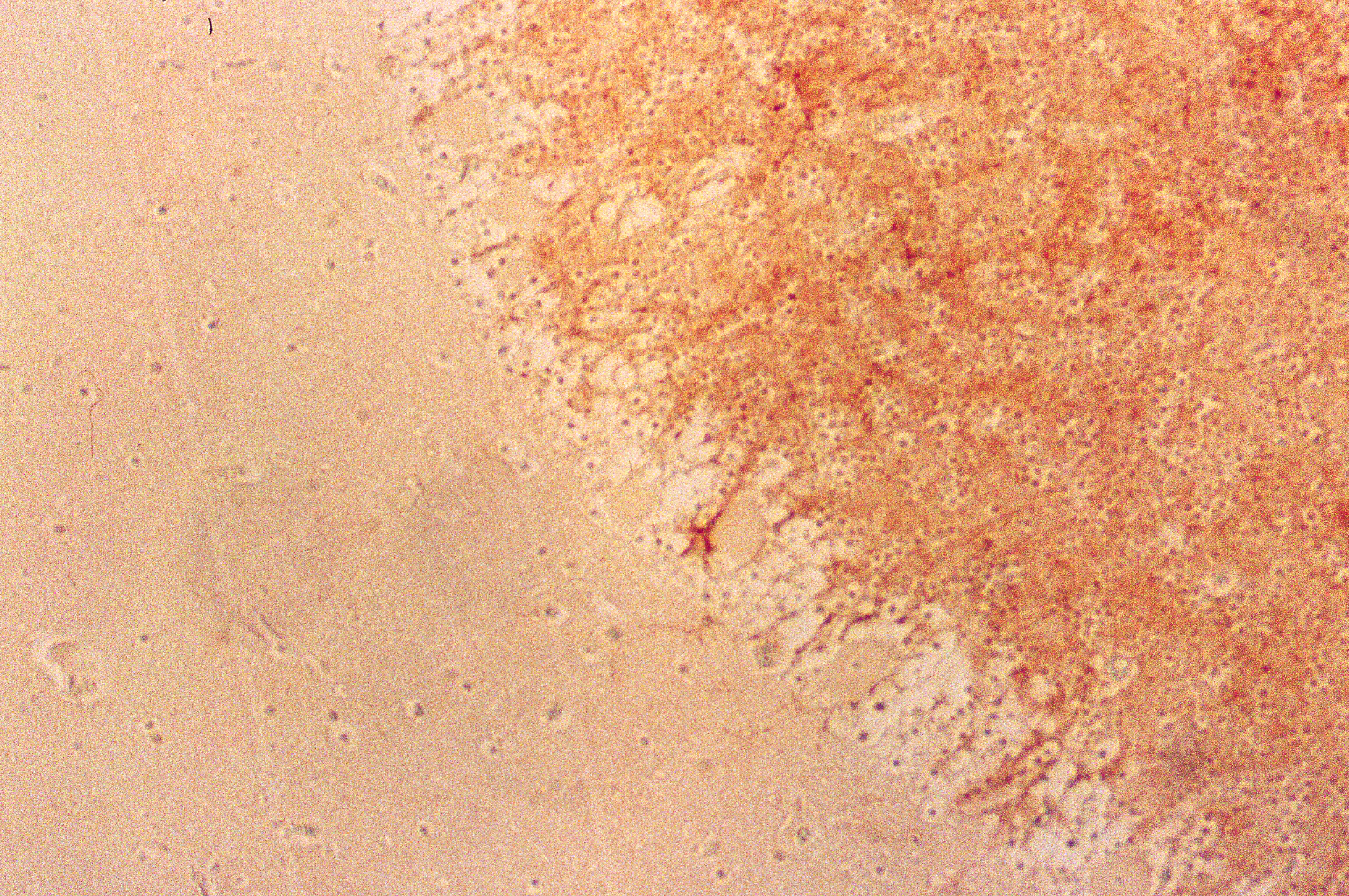
Results Antibody specificity Anti-Lewis x antibody stained clearly the granule cells in the blood vessels and showed intensive reactivity with the dendrites of granular cell layer in the cerebellum, microglia and astrocyte cells in the cerebellum and medulla oblongata. The results obtained by anti Lewis x were shown in Fig. 1. However, no reactivity was observed in the Purkinje cells and the neurons in the granular cell layer, the nucleus dentate of the cerebellum and the neurons in the nuclei of the medulla oblongata. There was no reactivity observed in these tissue sections by anti-sialyl-Lewis x antibody (10). On the other hand, anti-PGDS antibody stained the Purkinje cells and neurons of the nucleus dentate in the cerebellum and neurons in the nuclei of the medulla oblongata. Fig.1 Immunostaining (DAB) of anti-Lewis x antibody in human cerebellum and medulla oblongata.
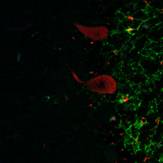
The result obtained by double immunofluorescent staining of the cerebellum with antibodies against Lewis x and PGDS is showed in Fig.2. The dendrites of the granular cell layer stained with anti-Lewis x antibody was shown in green color and the Purkinje cell was stained with anti-PGDS antibody and shown in red.
Fig. 2 Double immunofluorescent staining in human cerebellum.
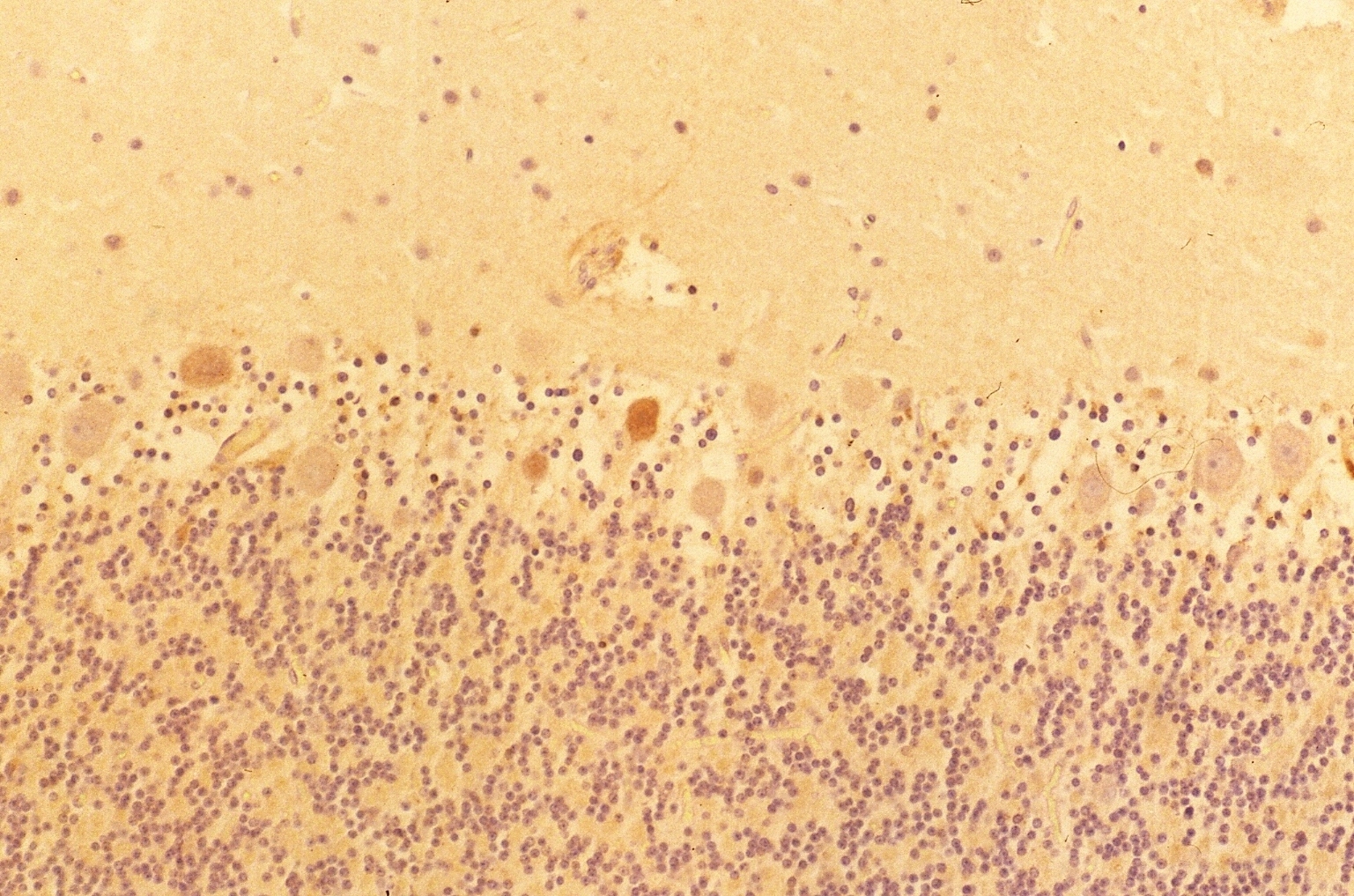
The results indicated that the staining patterns of these two antibodies were clearly different. Lewis x staining (green) was observed mostly in granular layer of the cerebellum(c) and PGDS staining (red) was observed in Purkinje cells in molecular layer, which can be presumed by their distinct shapes (b). The anti-PGDS antibody stained cytosol of the Purkinje cells but not in nuclei of the cell. Some of the cells in granular layer show positive reaction to PGDS and two overlapping reaction with Lewis x and PGDS resulted in yellow signal in (a). In Purkinje cells no green or yellow signals was observed at all, thus it can be said that anti-PGDS antibody does not recognize Lewis x and sialyl Lewis motif in the PGDS molecule. The distribution of PGDS in the cerebellum and medulla oblongata The Purkinje cells and the neuronal cells in the dentate nucleus (data not shown) in the cerebellum were clearly and intensively stained by the antibody (Fig. 3), although small amount of the Purkinje cells showed feeble or no reactivity with the antibody. The reactivity with anti-PGDS with the Purkinje cells was variable. Fig. 3 Immunostaining (DAB) of anti-PGDS antibody in cerebellum
The large neuronal cells in the nucleus olive (Fig. 4 c) and numerous neurons in other nuclei including nucleus nervi hypoglossi (Fig. 4 b), nucleus gracilis, nucleus cuneatus and nucleus ambiguous were clearly stained by anti-PGDS antibody, that is, all of the neuronal cells in the nuclei in the medulla oblongata showed intensive reactivity with the antibody, irrespective of the cell-shapes and the localization of neurons (Fig. 4 a). Fig. 4 Immunostaining (DAB) of anti-PGDS antibody in medulla oblongata
The reactive site with anti-PGDS antibody in the Purkinje cells, neurons in the dentate nucleus and nuclei in the medulla oblongata was localized in the cytosol and dendrite of the cells. No reactivity was observed in nuclei and membrane of these cells.
Fig . 5
Figure 5 shows a case of DAB staining with anti-PGDS antibody in cerebellum. This individual had suffered from acute ischemia, falling unconscious and been in vegetative state for months before he passed away. The granular layer and molecular layer were loosely attached along Purkinje cells. There are some Purkinje cells visible but only one showed positive reaction to the anti-PGDS antibody (left in Fig. 5). And two cells showed condensed staining of anti-PGDS antibody (middle in Fig.5). Other Purkinje cells show no reactivity to anti-PGDS antibody. We think this was due to the brain damage followed by ischemia and hypoxia and the cell with the condensed staining may be undergoing/have undergone the apoptotic changes through the period of the vegetative state. Although this was the known history of hypoxia, we can also determine whether or not there was a brain damage in cases with suspected hypoxia.
Discussion The results indicated that the antibody produced in our laboratory was specific to PGDS and not specific to the carbohydrate antigens such as Lewis x and sialyl Lewis x and also paraffin embedded tissue sections was able to be utilized for fluorescent immunohistochemistry. The PGDS catalyzes the isometric conversion pf PGH2 to PGD2. There are two types of PGDS, lipocalin-type PGDS (L-PGDS) and hematopoietic PGDS, and prostaglandin J2 (PGJ2) was derived by dehydration of PGD2 (13), indicating that both of PGD2 and PDJ2 may be expected in the co-working in the area where PGDS is detected. Berchtold-Kanz et al (16) reported that electro-convulsive shock evoked the release of only PGD among prostaglandins from the rat cerebellum, and Siggin et al (17) reported that 15-OH-PG dehydrogenase activity was detected in the molecular and Purkinje cell layers of rat cerebellum. According to these reports and the presence of PGDS in the Purkinje cells suggests that the Purkinje cells produce PGD and PGJ within the cells when the cells receive the stimuli therefore it can be said that PGDS in the Purkinje cells may play significant roles in the neurophysiological function. Since it is well known that the Purkinje cells are quite sensitive to brain ischemia (18), hypoxia (19) and heat stroke (20), and Dore-Duffy et al (21) reported that exposure of primary rat central nervous system (CNS) microvascular pericyte to low oxygen in vitro induced the rapid synthesis and release of the cyclopentenone PGD2 and PGJ2 within 15-30 minutes following hypoxic stress signal, thus anti-PGDS antibody might become useful tool for the detection of viability of the Purkinje cells and determination for the assessing the brain damage due to ischemia , obstructive sleep apnea and/or heat stroke in cadavers. In this study the antibody to PGDS intensively stained the Purkinje cells, however, some of the cells showed no reactivity. Since there have been many studies on the localization of neuroactive substances in the Purkinje cells, indicating the existence of chemical heterogeneity of the Purkinje cells (22), the presence of the cells with no reactivity to the antibody may be due to the chemical heterogeneity or damage (18,19,20) of the Purkinje cells. Although many studies have attempted to clarify the biological activities of L-PGDS/PGD in the CNS, there is little information on the factors governing the synthesis and release of PGs. In this study the reactivity of anti-PGDS antibody was observed in the cerebellum and medulla oblongata. The Purkinje cells receive information via two distinct afferent systems: the mossy fiber-parallel fiber pathway and the climbing fiber pathway. These two pathways each generate a distinct postsynaptic effect on the Purkinje cell. The deep cerebellar nuclei project not only to the cerebral cortex and to various motor centers in the brainstem, but also to the source of the climbing fiber input to the cerebellum and the inferior olive. There are also reciprocal olivo-nuclear connections (23). Since the cerebellum and medulla oblongata, in addition to their functions in equilibrium, motor tone and movement coordination, play critical roles in controlling blood pressure, respiratory patterning and sleeping (15, 24), PGDS that is major protein in the CSF and produces PGD might play an important and fundamental roles in the brain functions.
It is known that cells in cerebellum are susceptible to hypoxia, especially Purkinje cells. As they are constantly active and their demand on oxygen is also constantly high, a subtle hypoxia can affect their functions. This can be caused by suffocation, sub-acute hypoxia due to sleep apnea. Therefore we can use this antibody to evaluate the viability of Purkinje cells in cerebellum, determining whether there had been the state of hypoxia in cadavers or not. In this study we showed that Purkinje cells show heterogeneity in reacting with anti-PGDS antibody, this might be due to their variability in functions. In other words, we observed cells in different stages in their activities in one section of the tissue. We might be able to use this antibody to diagnose and to determine the cause and mechanisms of the suffocation, sleep apnea and also Sudden Infant death Syndrome. Acknowledgements This work was supported in part by a Grant-in Aid for Scientific Research (No.15390212 to K.N.) from the Ministry of Education, Culture, Sport, Science and Technology, Japan. References 1. Hata A N, Breyer R M. Pharmatocology and sigbaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharamacology Therapeutics. 2004; 103:147-166. 2. Abdel-Halim MS, Hamberg M, Sjoequist B, Awnggard E.Identification of prostaglandin D2 as a major prostaglandin in homogenates of rat brain. Prostaglandins. 1977;14:633-643. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=594367&query_hl=2 3. Ueno R, Honda K, Inoue S, Hayaishi O. Prostaglandin D2, a cerebral sleep-inducing substance in rats. Proc Natl Acad Sci USA. 1983; 80: 1735-1737. 4. Ueno R, Narumiya S, Ogorochi T, Nakayama T, Ishikawa T, Hayaishi O. Role of prostaglandin D2 in the hypothermia of rats caused by bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1982; 79: 6093-6097. 5. Kinoshita F, Nakai Y, Katakami H, Imura H, Shimizu T, Hayaishi O. Suppressive effect of prostaglandin(PG)D2 on pulsatile luteinizing hormone release in conscious castrated rats. Endocrinology. 1982; 110: 2207-2209. 6. Watanabe Y, Tokumoto H, Yamashita A, Narumiya S, Mizuno N, Hayaishi O. Specific bindings of prostaglandin D2, E2 and F2 alpha in postmortem human brain. Brain Res. 1985; 342: 110-116. 7. Hoffmann A, Conradt HS, Gross G, Nimtz M, lottspeich F, Wurster U. Purification and chemical characterization of beta-trace protein from human cerebrospinal fluid: its identification as prostaglandin D synthase. J Neurochem, 1993; 61:451-456. 8. Urade Y, Kitahama K, Ohishi H, Kaneko T, Mizuno N, Hayaishi O. Dominant expression of mRNA for prostaglandin D synthase in leptomeninges, choroids plexus and oligodendrocytes of the adult rat brain. Proc Natl Acad Sci USA. 1993: 90; 9070-9074. 9. Bloedorn B, Maeder M, Urade Y, Hayaishi O, Felgenhauer , Bruck W. Choroid plexus: the major site of mRNA expression for the beta-trace protein(prostaglandin D synthese) in human brain. Neurosci Lett. 1996; 209:117-120. 10. Grabenhorst E, Nimtz M, Costa J, Conradt HS. In vivo specificity of human alpha 1.3/4-fucosyltransferase III-VII in the biosynthesis of Lewis x and syalyl Lewis x motifs on complex-type N-glycans. Coexpression studies from BHK-21 cells together with human beta-trace protein. J Biol. Chem.1998; 273:30985-30994. 11. Ushiyama I, Nishimura A, YamamotoY, Yamada Y, Nagatomo M, Nishi K, Tanegashima A., The expression of Blood group ABO and related antigens in the human hypophysis. In 6th Indo pacific congress on Legal Medicine and Forensic Science, INPALMS-1998-Kobe, Ed by Tatsuno Y, Published by Committee of INPALMS. 1999; pp964-967 12. Yamamoto Y, Nakaminami C, Nakagawa T, Yamamoto A, Ohkubo I, Nishi K. Purification of basophilic low molecule protein from cerebro-spinal fluid and its biochemical characteristics. Jpn J Legal Med. 2004; 58:57 (in Japanese) 13. Nagasaka T, Hirade M, Sugimoto T, Shindo K, Shiozawa Z, Yokota S. Localization of lipocain-type prostaglandin D synthase in rat brain: immunoelectron microscopic study. Histocem Cell Biol. 2004; 121: 483-491. 14. Urade Y, Hayaishi O. Prostaglandin D2 and sleep regulation. Biochim Biophys Acta. 1999; 1436:606-615. 15. Bremer F. L’activite cerebral au cours du sommeil et de narcose: contribution a l’etudedu mecanisme du sommeil. Bull Acad R MedBelg.1937; 2:68-86. 16. Berchtold-Kanz E, Anhut H, Heldt R, Neufang B, Hertting G. Regional distribution of arachidonic acid metabolites in rat brain following convulsive stimuli. Prostaglandins 1981:22: 65-79. 17. Siggins G, Hoffer B, Bloom F. Prostaglandin-norepinephrin interactions in brain: microelectrophoretic and histochemical correlates. Ann NY Acad Sci. 1971; 180:302-323) 18. Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O’Hearn E, Molliver ME, Aicher SA. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331-359. 19. Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neuroscience Letter 2005;375-123-128. 20. Bazille C, Megarbane B, Bensimhon D, Lavergne-Solove A, Baglin AC, Loirat P, Woimant F, Mikol J, Gray F. Brain damage after heat stroke. J Neuropathol Exp Neurol. 2005;64:970-975. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16254491&query_hl=1 21. Dore-Duffy P, Balabanov R, Beaumout T, Katar M. The CNS pericyte response to low oxygen: early synthesis of cyclopentenone prostaglandins of the J-series. Microvasc Res. 2005; 69: 79-88 22. Cha-Palay V. Nilaver G, Palay S L, Beinfeld M C, Zimmerman E A, Wu JA, O’Donoue TL. Chemical heterogeneity in cerebellar Purkinje cells: Existence and coexistence of glutamic acid decarboxylase-like and motilin-like immunoreactivities. Proc Natl Acad Sci USA, 1981: 78:7787-7791 23. De Zeeuw CI, van Alphen AM, Hawkins RK, Ruigrok TJ. Climbing fibre collaterals contact neurons in the cerebellar nuclei that provide a GABAergic feedback to the inferior olive. Neuroscience 1997; 80: 981-986. 24. Garifoli A, Scardilli G, Perciavalle V. Effects of cerebellar dentate nucleus GABAergic cells on rat inferior olivary neurons. Neuroreport 2001; 12:3709-3713. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11726779&query_hl=33
|
|
Please return again frequently as we have some rather BIG PLANS ahead for our web site. We intend to be so much more than just a peer reviewed online journal of medicine. Once completed our web site will contains viable articles, expert opinions, letters, and extensively researched reports. All articles may be submitted by e-mail as well as by post and will be evaluated accordingly. We thank you for your patience. |
|
|
|
Thorax.us makes
absolutely no representation as to the content, accuracy, or
completeness of the information contained on this web site or the linked
web sites and does not necessarily endorse any of the information
contained therein. All readers and/or patients should verify all of the
information and data before ever administering any type of drug, therapy
or treatment discussed herein. Neither the web designers, editors nor
the publisher accepts any responsibility for the accuracy of the
information or the consequences from the use or misuse of the
information contained herein. Information on this web site is provided
for informational purposes only and is not a substitute for professional
medical advice. You should not use the information on this web site for
diagnosing or treating a medical or health condition. You should
carefully read all product packaging. If you have or suspect you have a
medical problem, promptly contact your professional healthcare provider.
Statements and information regarding supplements have not been evaluated
or approved by the Food and Drug Administration. Please consult your
healthcare provider before beginning any course of supplementation or
treatment. |
|
Copyright © 2005 Thorax.us All Rights Reserved |
 (a)
(a)

 (a)
(a) 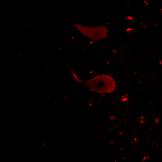 ( b)
( b) 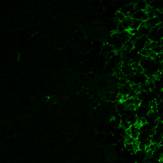 (c)
(c)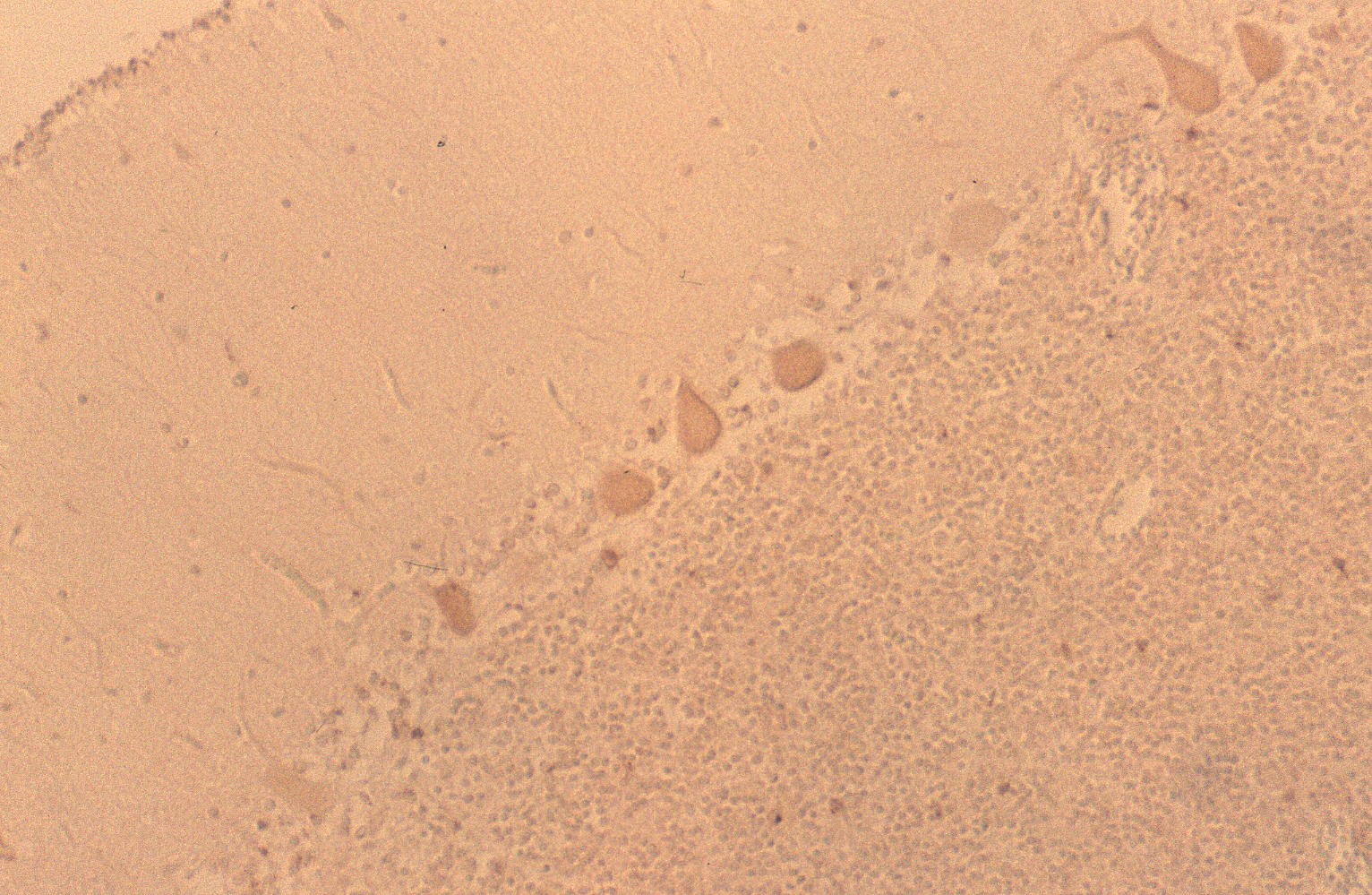 (a)
(a) 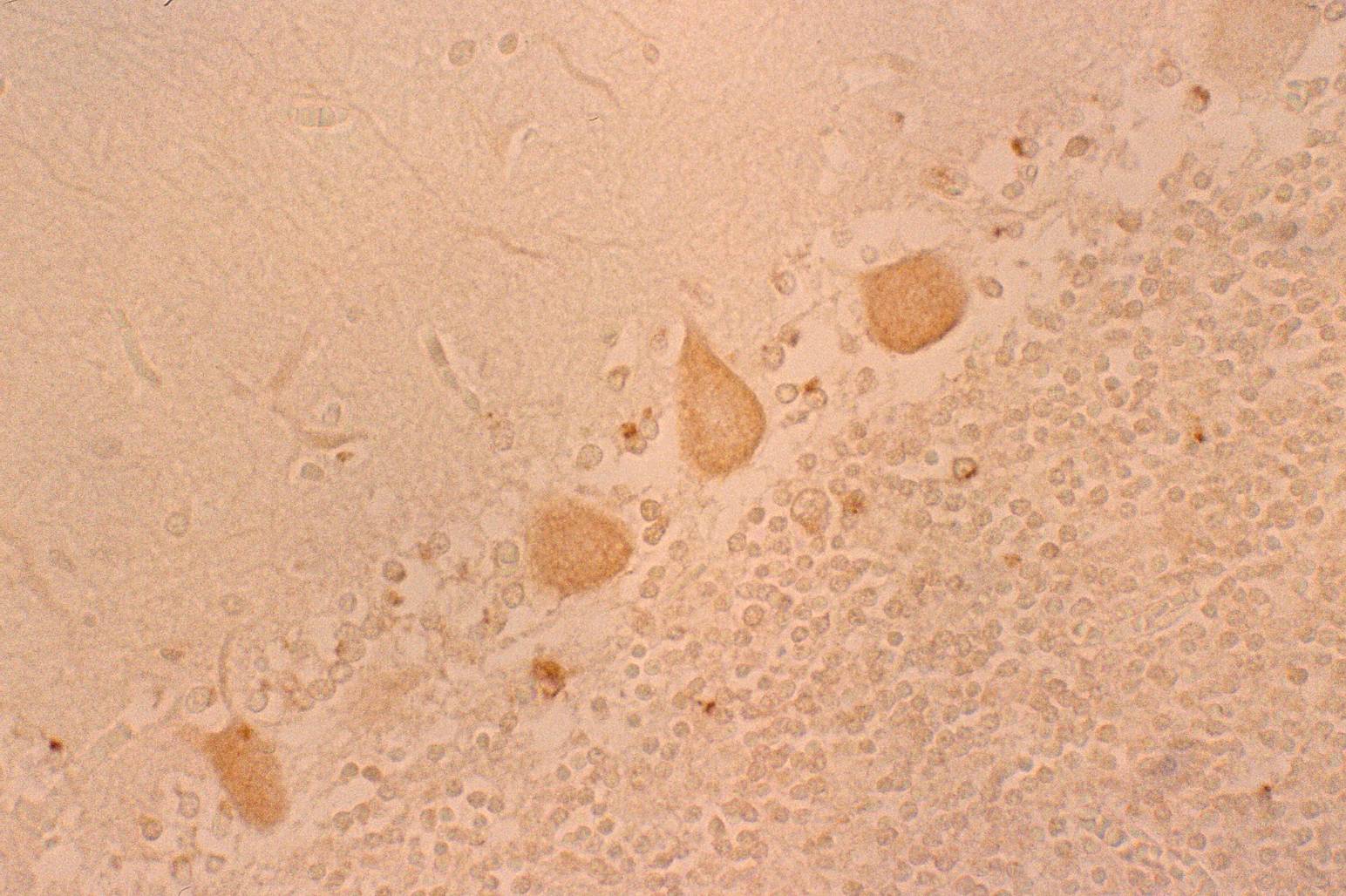 (b)
(b)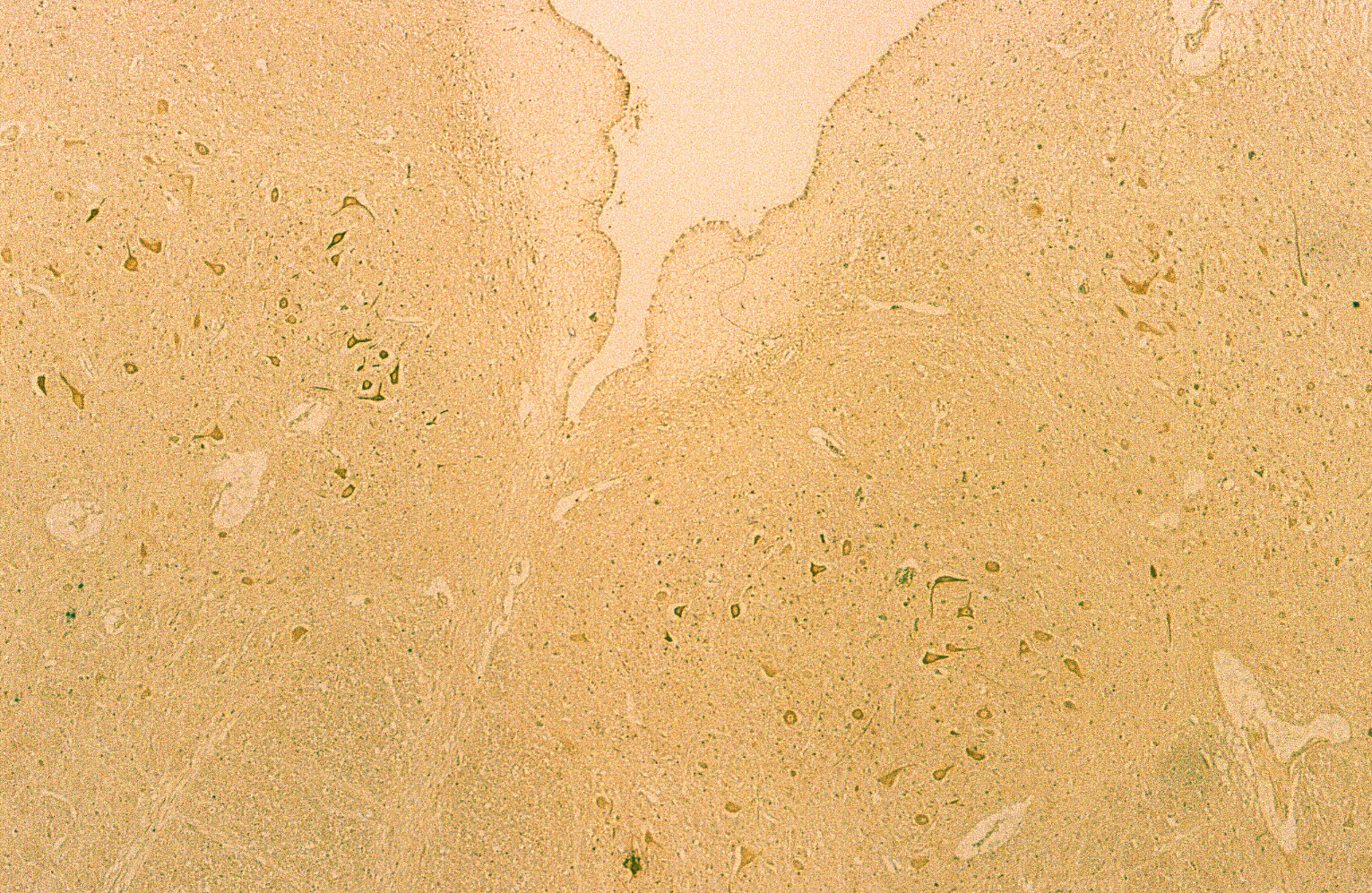 (a)
(a) 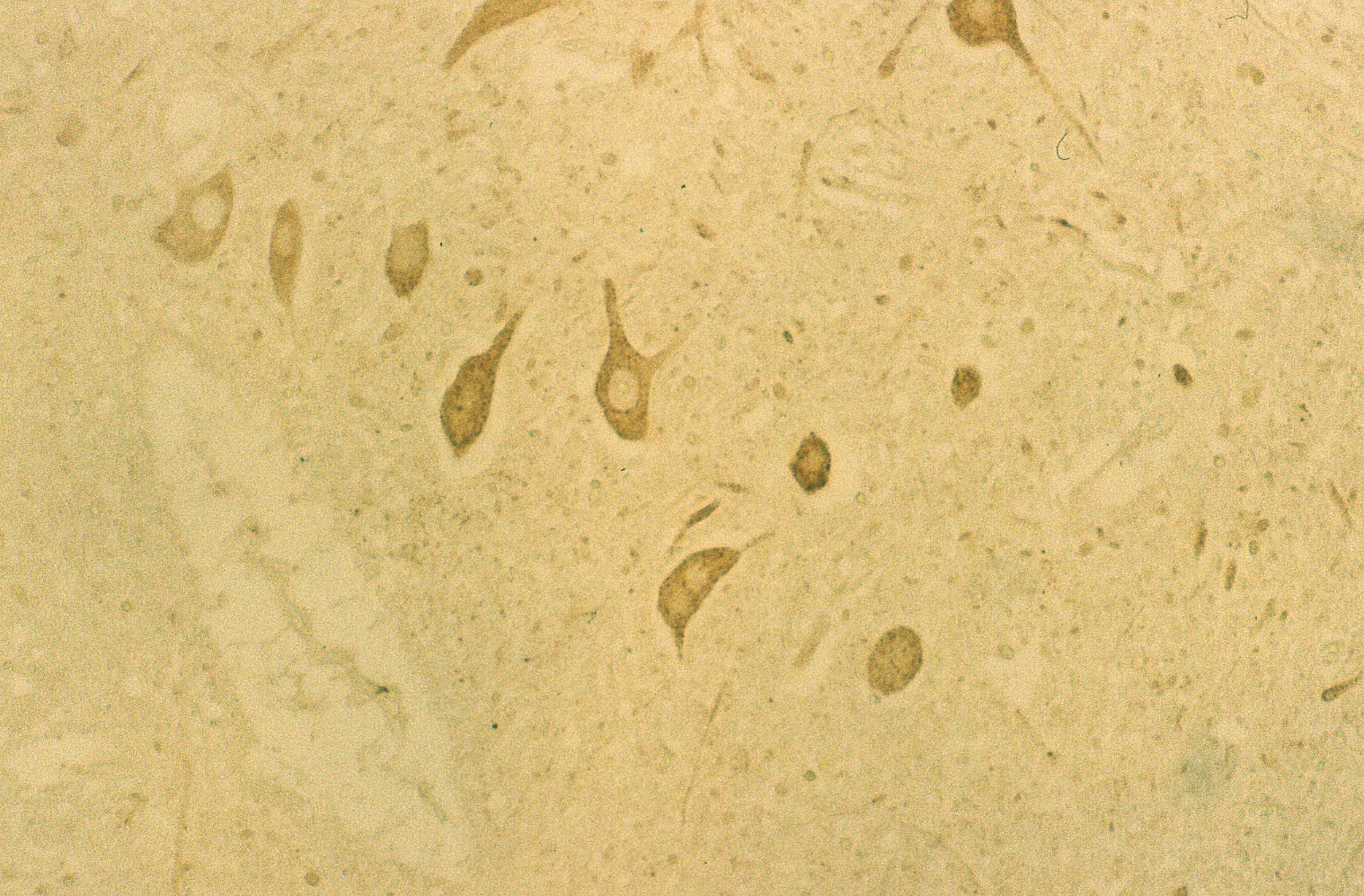 (b)
(b) 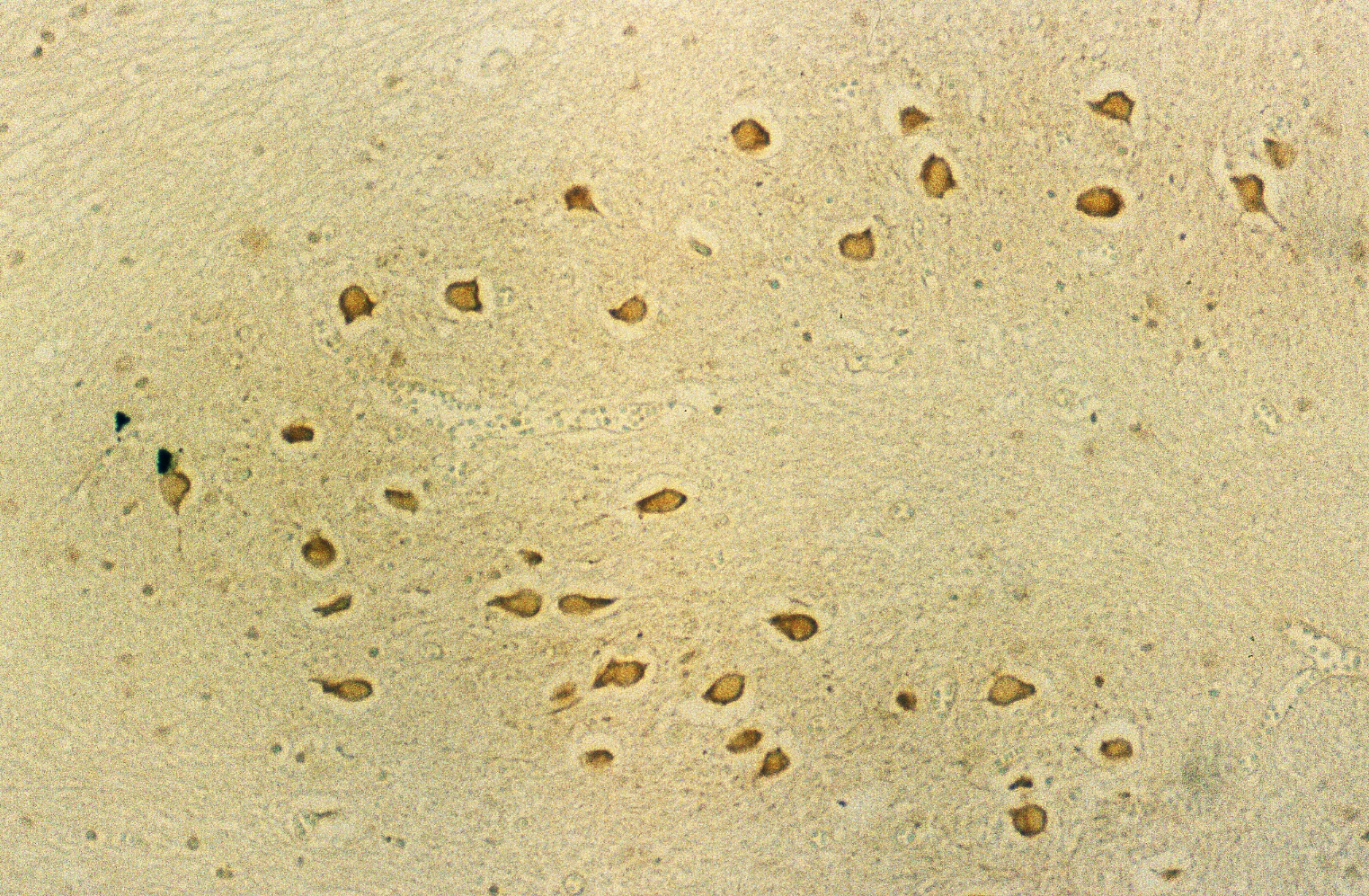 (c)
(c)